Parallel evaporation is a crucial technique in the field of laboratory science that facilitates the rapid and efficient concentration of multiple samples simultaneously. This topic holds immense importance due to its wide range of applications in various research areas, such as drug discovery, combinatorial chemistry, and analytical chemistry. Parallel evaporation enables scientists to accelerate sample processing, remove solvents, and concentrate target compounds, enhancing efficiency and productivity.
By discussing parallel evaporation, we delve into the principles, methodologies, and advancements in this technique. Therefore, exploring the topic of parallel evaporation is essential for researchers seeking to streamline sample preparation, improve analytical outcomes, and advance scientific discoveries.
Definition of concentration in chemistry
Concentration procedure and methods
Evaporation concentration
Parallel evaporation / Vacuum-vortex evaporation
Comparison of evaporation technologies
Definition of concentration in chemistry
In chemical analysis, concentration is a sample pretreatment process used to separate the solvent and solute by removing the solvent from the solution. This process aims to increase the concentration of the solute. Concentration allows for an increase in the ratio of the target substance in a solution, either in relation to the solvent or the total solution. By reducing the factors that may affect experimental results, concentration serves as an essential step in sample pretreatment before analysis.
Concentration procedure and methods
Concentration procedure can be described as follow:
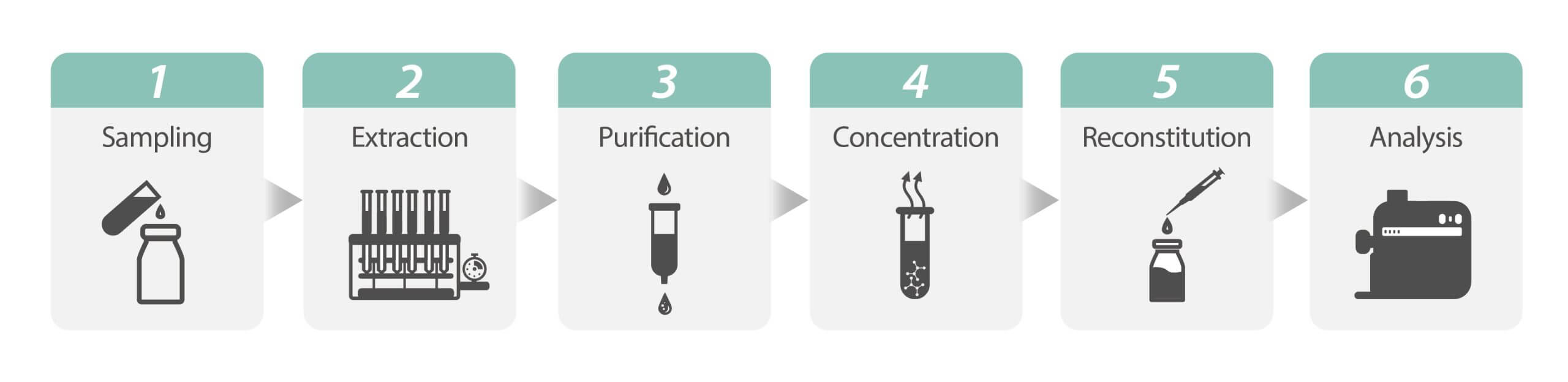
- Sampling: After homogenizing the sample, take an appropriate amount of samples according to the experimental method.
- Extraction: Separate the target substances from mixtures and obtain an extract. Common extraction methods include solid/liquid extraction, liquid/liquid extraction, solid-phase extraction (SPE), solid-phase micro-extraction (SPME), supercritical fluid extraction (SFE), and immunoaffinity column (IAC).
- Purification: Use physical or chemical methods to remove impurities from the extract. The most common purification method is solid-phase extraction (SPE).
- Concentration: Increase the ratio of the target substance in a solution and enhance sample sensitivity.
- Reconstitution: Restore the concentrated samples to a liquid state using solvents.
- Analysis: Analyze the restored liquid sample using instruments such as HPLC (High-Performance Liquid Chromatograph), GC (Gas Chromatograph), LC-MS/MS (Liquid Chromatograph/Tandem Mass Spectrometer), or GC-MS/MS (Gas Chromatograph/Tandem Mass Spectrometer).
Concentration procedure example: An LC‐MS/MS Method for the Determination of Antibiotic Residues in Distillers Grains (C-012.01), FDA
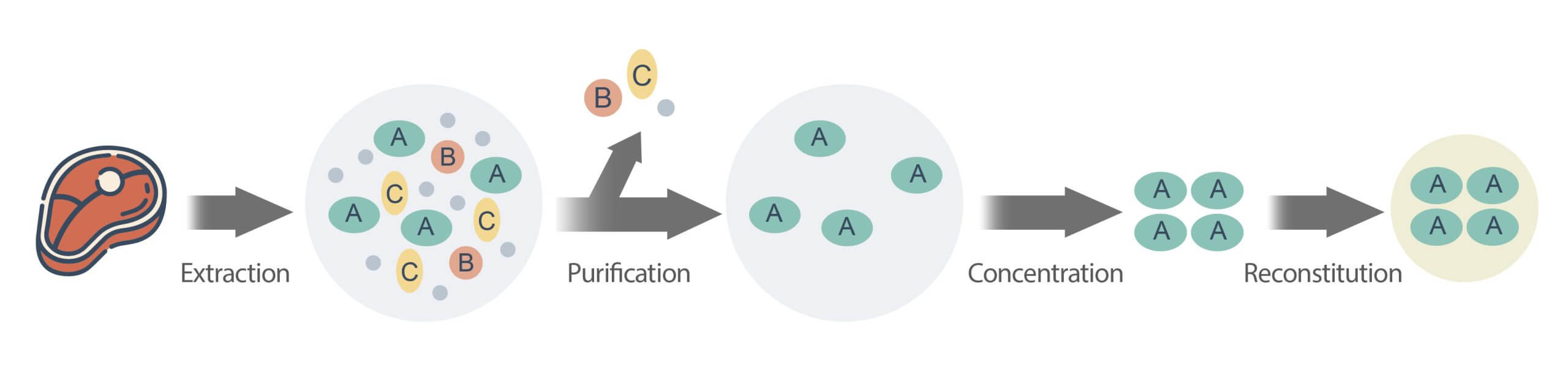
- Sampling: Weigh 5.0 ± 0.1 g ground distillers grains into individual 50 mL tubes.
- Extraction: Add acetate buffer and acetonitrile (ACN) for extraction.
- Purification: Use Solid Phase Extraction on Oasis HLB (60 mg, 3 mL) to purify the extract.
- Concentration: Evaporate eluates at 40 oC to 50 µL.
- Reconstitution: Reconstitute with 80:20 mixture of water ACN and filter with membrane.
- Analysis: Analysis antibiotic residues by LC-MS/MS.
Evaporation & concentration
There are several methods of concentration, such as evaporation, membrane concentration, and freeze concentration. The principle of evaporation in concentration involves the removal of the solvent. As the liquid is vaporized and removed as a gas, less volatile samples are left concentrated and free from the solvent, resulting in a dry and solvent-free state.
Common evaporation techniques
Common concentration techniques used in laboratories include rotary evaporation, nitrogen evaporation, centrifugal evaporation, and vacuum-vortex evaporation.
Rotary evaporation
Rotary evaporation techniques promote solvent evaporation by rotating a round bottom flask at an elevated temperature and under reduced pressure. To perform rotary evaporation, five components are required: a heat bath, rotor, condenser, solvent trap, and a vacuum pump. However, a major disadvantage of rotary evaporation is its limited capacity to process only one sample at a time.
Nitrogen blowdown evaporation
Nitrogen blowdown evaporators utilize thin tubes (needles) to blow a continuous stream of nitrogen gas onto the surface of a solvent, thereby reducing the vapor pressure and increasing the surface area for faster evaporation. The process can be expedited by applying a heating block or dry bath to facilitate solvent vaporization. Nitrogen evaporators are commonly employed for small sample volumes (less than 50mL). However, caution must be exercised to manage the risk of cross-contamination since it utilizes open vials. Additionally, it may not be the most suitable method for removing less volatile solvents.
Centrifugal evaporation
The principle of centrifugal evaporation is based on reducing pressure using a vacuum to induce solvent boiling. Heat energy, typically from infrared or steam, is also applied to accelerate evaporation. Centrifugal force ensures that the solvent boils from the sample surface downwards, minimizing the risk of boiling over, solvent bumping, sample loss, and cross-contamination. Centrifugal evaporation is an ideal technique for processing multiple samples with smaller volumes. However, it is not possible to make observations during the process due to the enclosed nature of the centrifuge tubes.
Parallel evaporation / Vacuum-vortex evaporation
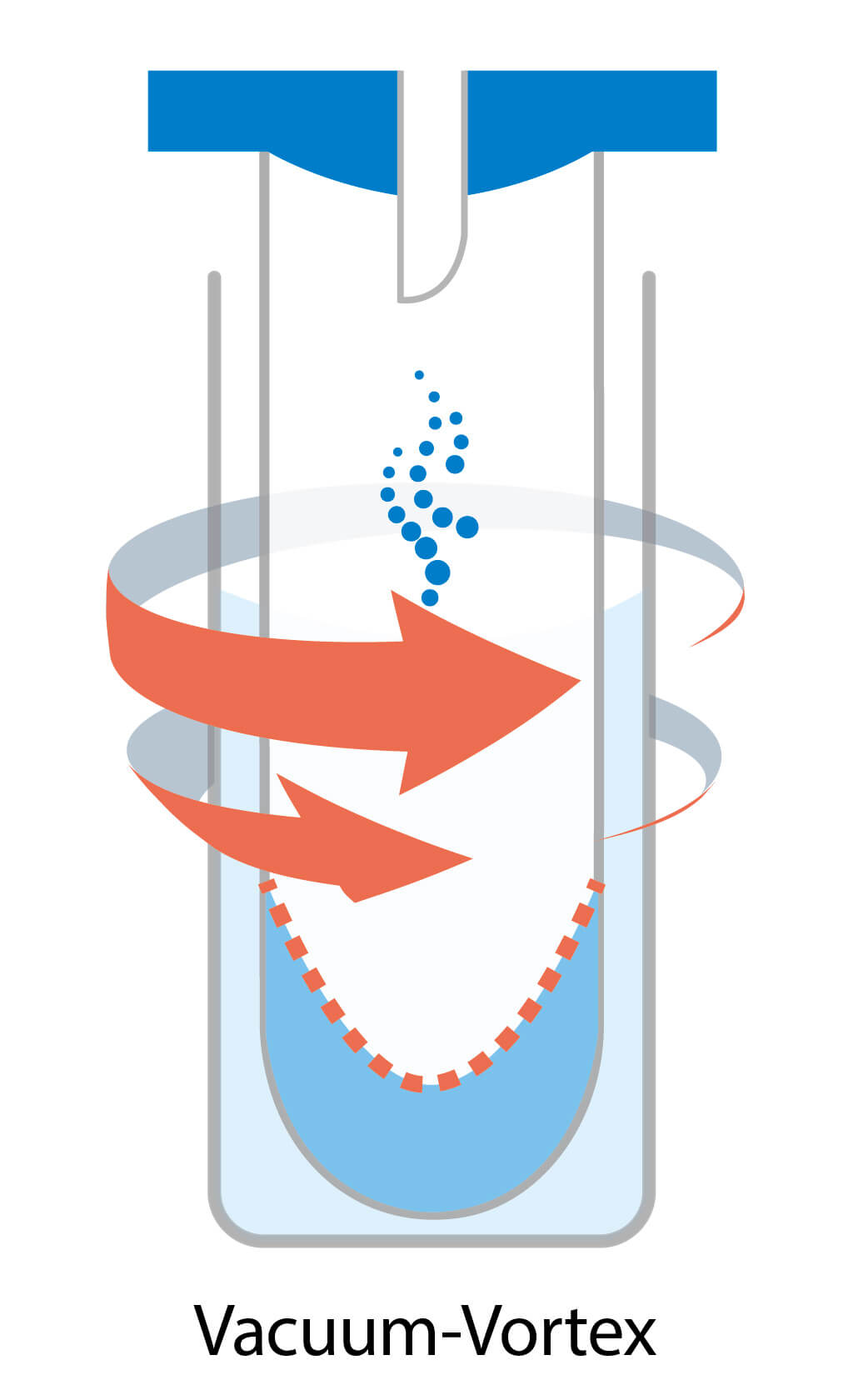
Parallel evaporation involves creating a vortex by swirling the sample tubes, which leads to an increased sample surface area and faster vaporization. However, the centrifugal force generated by the vortex is often insufficient to prevent bumping, resulting in a higher risk of sample loss and cross-contamination compared to centrifugal evaporation.
To address these challenges, more advanced vortex evaporators incorporate vacuum pumps to reduce pressure and heaters to increase temperature, further accelerating the evaporation process. Additionally, a cold trap is essential in such systems to collect solvent gases and prevent them from escaping.
By combining vacuum pressure, heating, and a cold trap, advanced vortex evaporators aim to improve the efficiency of the evaporation process while minimizing sample loss and cross-contamination.
Comparison of parallel evaporator with other concentration methods
|
|
Lab A |
Lab B |
Lab C |
|||||
|
Test |
Pesticide residues in food |
Antibiotic Residues in Animals |
Biochemical extraction |
|||||
|
Sample size |
48 |
12 |
12 |
|||||
|
Solvent used |
1 mL ACN |
50 mL Acetate buffer and acetonitrile (ACN) |
1 mL sterile water |
|||||
|
Concentration Technique |
Nitrogen Concentration |
Parallel Evaporator |
Vacuum Concentration |
Nitrogen Concentration |
Parallel Evaporator |
Centrifugal Vacuum Concentration |
Nitrogen Concentration |
Parallel Evaporator |
|
Procedure time |
40 mins |
10 mins |
4 hours |
2 hours |
1.5 hours |
200 mins |
65 mins |
20 mins |
Comparison of evaporation technologies
|
Concentration Technologies |
Vacuum Vortex Concentration/ Parallel Evaporator |
Rotary Concentration |
Nitrogen Blowdown Concentration |
Centrifugal Concentration |
|
|
||||
|
Number of Sample |
Multiple (12 / 48) |
1 |
Multiple |
Multiple |
|
Parameter Controls |
Vacuum, temperature, RPM, time |
Temperature, RPM |
Temperature, flow speed |
Temperature, RPM, time |
|
Cross-contamination |
Risk-free |
Risk-free |
High risk |
Low risk |
|
Consumable parts |
No |
No |
Nitrogen |
No |
| Visibility |
Yes |
Yes |
Yes |
No |
|
Tube Loading/ Unloading |
Batch & single |
Single |
Batch & single |
Single |
|
Solvent Vapor Collection |
Yes. In-room. |
Yes. In-hood. |
No. In-hood. |
Yes. In-room. |
Common Applications of Parallel Evaporator
- Food analysis, including veterinary medicine, pesticide residue.
- Environmental testing, including Environmental agents, Diosin.
- Sample extraction, concentration and evaporation clinical experiments, biology, chemistry….etc.
References:
Centrifugal concentrators for biological applications.
Types of Solvent Evaporators – An Overview
NITROGEN EVAPORATORS (Sample Concentrators)
Introduction to Evaporation – biophara group
Evaporators – American Chemical Society
Deciding Whether or Not to Use a Fume Hood With Your Rotary Evaporator