Rotary evaporation is a common technique that’s widely used in laboratory settings for efficient solvent removal and concentration. It holds diverse applications in fields such as organic chemistry, pharmaceutical research, and food analysis. By utilizing gentle heat, vacuum, and rotation, a rotary evaporator, or rotovap, allows for the precise separation of solvents from samples, enabling the isolation of desired compounds.
This article covers insights into principles, operation, and advancements, which contribute to the purification of substances, extraction of volatile components, and sample preparation using rotary evaporation for further analysis.
What is a rotary evaporator
Advantages & disadvantages of rotary evaporation
Common evaporation techniques
Essential devices for rotary evaporation
Equipment required to perform rotary evaporation
Applications of rotary evaporator Rotary Evaporation VS Short-Path Distillation
What is a rotary evaporator
A rotary evaporator, also known as a rotovap or rotavap, is a commonly used device in chemical laboratories for the isolation and distillation of large quantities of a single sample. The principle of rotary evaporation is to distill volatile solutions by heating and increasing the surface area available for distillation. To enhance the efficiency of the process, a rotary evaporator is typically connected to a vacuum pump to create a vacuum, thereby reducing the boiling point of the solution. It is also recommended to add a condenser to collect the gases produced during distillation and prevent their emission, as these gases may be hazardous.
The principle of a rotary evaporator is that as the vacuum in the evaporating flask increases, the boiling point of the liquid inside decreases. With a sufficiently low vacuum level, even high-boiling point solvents such as water (boiling point at standard atmospheric pressure 100°C), dimethylformamide (153°C), and dimethyl sulfoxide (189°C) can be distilled. For example, by reducing the vacuum from 760 torr to 5 torr, both dimethylformamide and dimethyl sulfoxide can be made to boil at 50°C.
In most cases, rotary evaporators are used to separate low-boiling substances, such as hexane and ethyl acetate, which are liquids at room temperature and pressure. It is also possible to selectively remove specific substances from samples using a rotary evaporator.
Advantages & disadvantages of rotary evaporation
Advantages
As a concentration method that utilizes reduced air pressure, a rotary evaporator offers advantages compared to concentration using non-rotary containers. These advantages include the following:
-
Quicker: Due to the inertia and friction between the liquid and the rotating flask, the liquid spreads across the inner surface of the flask, forming a thin liquid film. This increased surface area facilitates more efficient distillation, resulting in faster concentration.
-
Reduced bumping: The liquid film mentioned earlier also helps to prevent bumping during the procedure. By maintaining a uniform and continuous liquid film, the likelihood of sudden bursts or violent boiling, which can lead to sample loss or contamination, is minimized.
Disadvantages
- The structure of rotary evaporators is difficult to clean and sanitize. Thus, when bumping occurs, it’s hard to avoid cross-contamination.
- Not suitable for foaming samples unless used with a defoamer or a specialized condenser.
- Distilled and concentrated substances will spread on the walls of the bottles.
Both ROCKER’s vacuum controllers are equipped with auto boiling point detection. Feel free to start and leave.
– DC Chem 610 Pro Auto Vacuum System
– Pilot 100 Vacuum Controller
ROCKER’s Vacuum Controllers
Common evaporation techniques
Common concentration techniques used in laboratories include rotary evaporation, nitrogen evaporation, centrifugal evaporation, and vacuum-vortex evaporation.
Rotary evaporation
Rotary evaporation techniques promote solvent evaporation by rotating a round-bottom flask at an elevated temperature and reduced pressure. To execute rotary evaporation, five components are required: a heat bath, rotor, condenser, solvent trap, and a vacuum pump. Its major disadvantage is the lack of capacity to process more than one sample at a time.
Nitrogen blowdown evaporation
Nitrogen blowdown evaporators use thin tubes (needles) to blow a continuous nitrogen gas stream onto the surface of a solvent, lowering the vapor pressure and increasing the surface area for evaporation. A heating block or dry bath can be applied to accelerate solvent vaporization. Nitrogen evaporators are often used for small volumes of samples (less than 50 mL). However, due to the use of open vials, the risk of cross-contamination should be carefully managed. It is also not the most ideal method for removing less volatile solvents.
The principle of centrifugal evaporation is to reduce pressure using a vacuum to induce solvent boiling. Heat energy, typically provided by infrared or steam, is also applied to accelerate evaporation. Centrifugation ensures that the solvent boils from the sample surface downwards, minimizing the risk of boiling over, solvent bumping, sample loss, and cross-contamination. Centrifugal evaporation is an ideal technique for processing multiple samples in smaller volumes. However, it is not possible to make any observations during the process.
Parallel evaporation / Vacuum-vortex evaporation
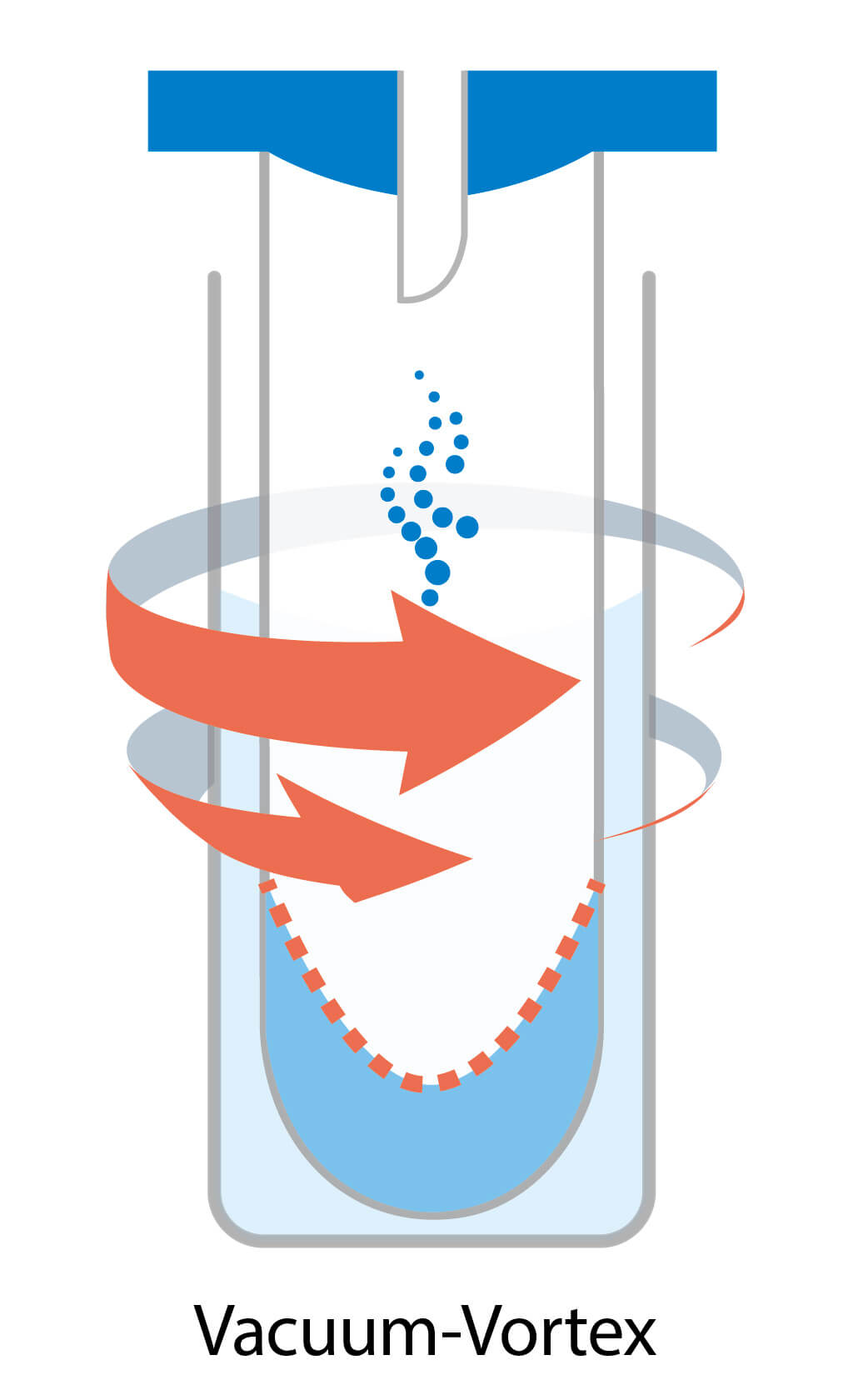
Parallel evaporation is a technique that involves creating a vortex by swirling the sample tubes. This vortex generates a larger sample surface, promoting vaporization and making the process faster. However, the g force generated to prevent bumping through the vortex is insufficient compared to centrifugal evaporation. As a result, typical vortex evaporators are prone to sample loss and cross-contamination. More advanced vortex evaporators incorporate vacuum pumps to reduce pressure and heaters to increase temperature, further accelerating evaporation. A cold trap is also essential in such a system to collect solvent gases.
Essential devices for rotary evaporator
Since the substances being processed in rotary evaporators mainly consist of chemicals and solvents, it is recommended to use chemical-resistant vacuum pumps made of polytetrafluoroethylene (PTFE). The vacuuming capacity of the pumps should be determined based on the solvent requirements, and the sizes should be selected based on the size of the sample or flask. Utilizing pumps with a vacuum controller can also provide flexibility in selection and ensure a more accurate and intelligent vacuuming procedure.
Key points to consider when deciding:
- Pump: Use a water-free and oil-free diaphragm vacuum pump with a low ultimate vacuum suitable for distilling high-boiling point solvents.
- Vacuum controller: Utilize a vacuum controller to help maintain the vacuum level or create a vacuum curve as needed.
- Sealing ring: PTFE material is commonly used for its high corrosion resistance when selecting a sealing ring.
- Cooling circulation system: Ensure that the cooling system remains at least 40°C lower than the temperature of the heating pot. Generally, a cooling circulation system is necessary to ensure efficient solvent recovery and maintain a safe and odor-free laboratory environment.
Equipment required to perform rotary evaporation
To perform rotary evaporation and concentration, you may need below items:
A. Rotary evaporator
Most rotary evaporators should include a sample rotating device, a heating water bath, a condenser, and a collector. To prevent potential hazards to the environment and human body, the condenser should be equipped with a circulation thermostat that has a cooling capacity at least 40°C lower than the temperature of the water bath. This temperature difference ensures an adequate vapor collection rate.
B. Vacuum pump
To decrease air pressure of the evaporation system and lower the boiling point of solvent.
Read more about: How to select proper vacuum pump for rotary evaporator
C. Vacuum controller and vacuum regulator
To control the system air pressure to ensure consistency, reproducibility and recovery yield.
- Manual control: Typically, it is controlled using a pressure regulating valve and a vacuum gauge, which is a rough control method.
- Vacuum controller: It provides precise pressure control through the use of a solenoid valve and motor speed. For separation of various mixed solvents, a model with a built-in multi-stage program can be selected. Some control systems include an automatic boiling point detection function, which determines the optimal vapor pressure of a solvent without requiring human intervention, making the operation easier.
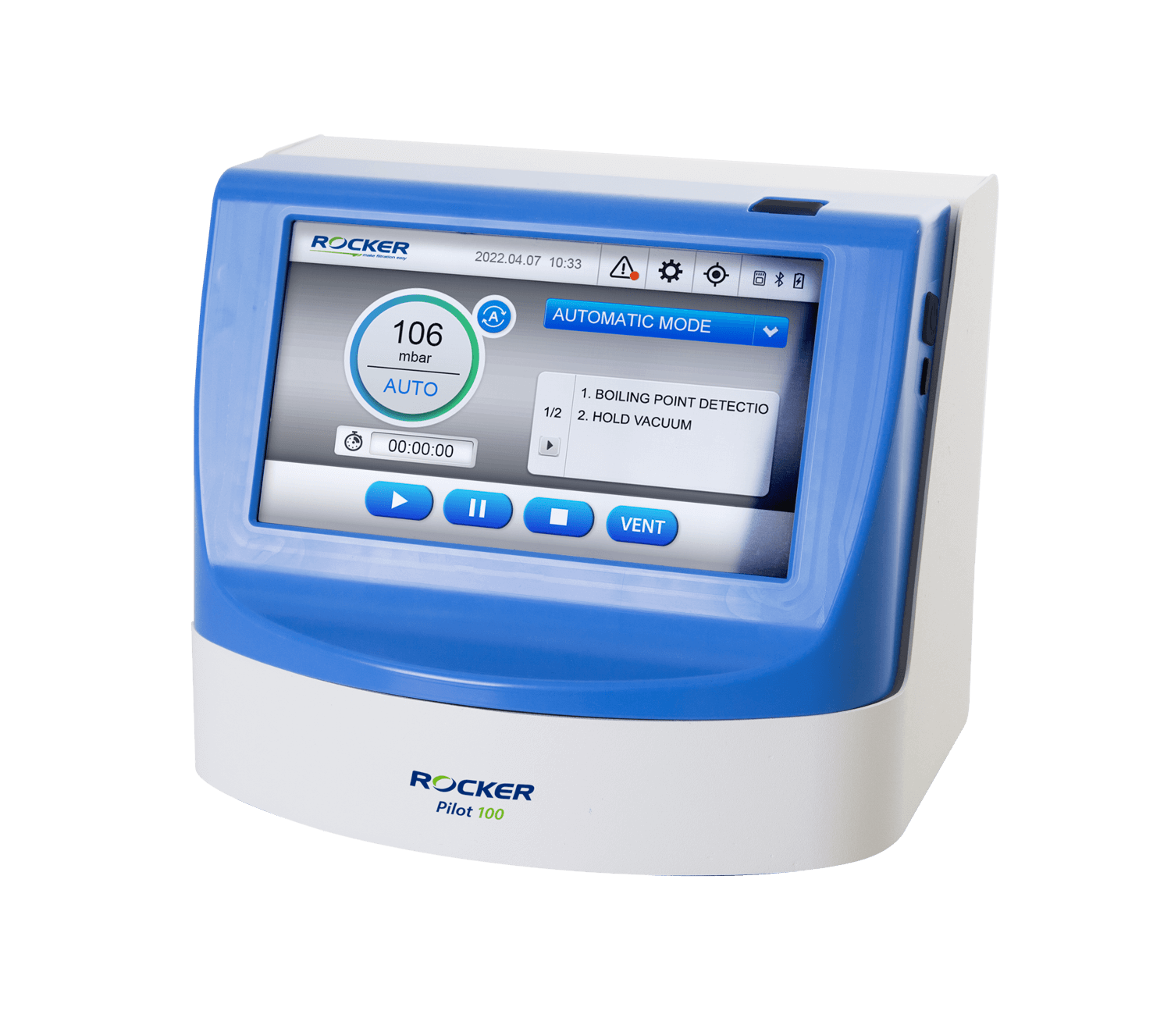
Pilot 100 Vacuum Controller
- Boiling point detection function to maintain the vacuum at the best state.
- Capacitive touch panel, easy to operate without taking off gloves.
Pilot 100 Vacuum Controller
Common applications of rotary evaporation
-
- Active ingredient development and extraction and concentration, such as Chinese herbal medicine, medical marijuana, cosmetics, skin care products, health care products, etc.
- Environmental testing, such as residual pesticides, environmental drugs, dioxins, environmentally harmful substances, total hydrocarbons in diesel water, etc.
- Food safety testing, such as animal drugs, pesticide residues, contraband, additives, etc.
Video: Pesticide Residue Analysis | Sample Preparation | Extraction and Cleanup | USEPA 3620C - Concentration, evaporation, purification experiments, such as biology, biotechnology, medicine, clinical, medical inspection, chemical industry, chemistry, etc.
</ul
Rotary Evaporation VS Short-Path Distillation
What is short-path distillation?
Short-path distillation, also known as molecular distillation, is a process that separates and purifies compounds by utilizing low-pressure evaporation based on differences in volatility. It typically separates 2 to 3 different substances and is therefore also referred to as fractional distillation. During distillation, a vacuum pump is usually used to apply negative pressure (vacuum) to the distillation system, lowering the boiling point or boiling pressure of the compounds. This helps facilitate evaporation at lower temperatures, reducing the risk of degradation and denaturation of heat-sensitive substances. Compared to other distillation methods, “short-path” distillation involves a short distance for the distillate to travel within the system, often just a few centimeters. This effectively reduces the exposure of the sample to high temperatures and minimizes sample loss, making it particularly suitable for the separation and purification of small quantities of substances. It is commonly used for heavier molecules and heat-sensitive compounds such as fuels, fatty acids, fragrances, and flavorings.
Rotary Evaporation VS Short-Path Distillation
|
|
Rotary Evaporation |
Short-Path Distillation |
|
Principle |
By reducing pressure to lower the boiling point, combined with heating and rotation to increase the volatile surface area, the evaporation of the liquid is accelerated. |
By reducing pressure to lower the boiling point, within a short distance, different compounds are separated and recovered based on their differences in volatility. |
|
Equipment required |
Rotary evaporator, or so-called rotovaps |
Short-path distillation devices like Kugelrohr and others. |
|
Solvent Type |
Simple mixtures, pure solvents |
Complex mixtures, such as hydrocarbons or other hydrocarbon compounds |
|
Cost of devices |
Lower |
Higher |
|
Power consumption |
Lower |
Higher, for it requires deeper vacuum. |
|
Technique requirements |
Lower |
Higher |
|
Application |
Concentration, separation, solvent removal, etc. |
Separation and purification of heat-sensitive, heavy molecules, etc. |
|
Industries |
Food, pharmaceuticals, chemical engineering, botanical extraction, etc. |
Chemical engineering, pharmaceuticals, essential oils, environmental analysis, etc. |
References: