Trace metal analysis is a crucial field of study that involves the detection, quantification, and characterization of trace levels of metals in various samples, including environmental samples, biological tissues, and industrial products. This topic holds immense importance due to the potential health risks and environmental implications associated with trace metal contamination. Analyzing trace metals allows for the assessment of pollution levels, identification of sources, and monitoring of metal concentrations in different matrices.
By discussing trace metal analysis, we gain insights into its methodologies, instrumentation, and advancements, which contribute to risk assessment, regulatory compliance, and the development of strategies to mitigate metal-related hazards.
What is trace metal
Trace metal analysis techniques
Acid digestion methods in trace metal analysis
Wet (acid) digestion methods
Comparison: CVD (closed vessel digestion) and OVD (open vessel digestion)
Most commonly used acids in metal digestion
What is trace metal?
Trace metals are usually referred to as a subset of metals under trace elements with a density of more than 5 g/cm3. Due to their chemical properties, trace metals are difficult to decompose and easily accumulate in food, soil, water, and the human body. Common trace metals that are regulated by food and drug regulations include total arsenic and inorganic arsenic, lead, cadmium, mercury and methylmercury, tin, copper, and antimony.
Trace metal analysis techniques
Before conducting trace metal analysis, samples should be digested into small molecules and then observed using instruments selected based on the methods. The most common methods for metal determination include:
- Flame Atomic Absorption Spectrophotometry, FAAS,
Ideal for observations of lead, cadmium, copper, antimony, tin and zinc. - Graphite Furnace Atomic Absorption Spectrometry, GFAAS
Ideal for observations of lead, cadmium, copper, antimony, arsenic, tin and zinc. - Inductively Coupled Plasma Atomic Emission Spectrometry, ICP-AES
Ideal for observations of lead, cadmium, copper, antimony, arsenic, mercury, tin and zinc. - Inductively Coupled Plasma Mass Spectrometry, ICP-MS
Ideal for observations of lead, cadmium, copper, antimony, arsenic, mercury, tin and zinc.
In order to obtain reliable results, pre-treatment should be carefully handled, considering the reagents and instruments specific to the selected observation method.
Acid digestion methods in trace metal analysis
Sample preparation for trace metal analysis involves metal digestion, which aims to destroy the sample matrix by adding an acid (oxidizing agent) and treating it with heat. This process removes unwanted components, leaving only the target analyte and achieving homogenization and pre-concentration.
However, the digestion process is time-consuming and poses hazards to analysts and equipment due to the corrosive vapors generated by the heated acid. Additionally, prolonged heating can lead to the loss of elements and affect the reliability of the results. Unfortunately, shortening the heating time is not an option as it may result in incomplete digestion.
Metal digestion methods include Dry Ashing and Wet Digestion.
Dry Ashing involves using a muffle furnace capable of maintaining temperatures of 500-600°C to ignite and oxidize organic compounds. The resulting carbonaceous residues (ashes) are then dissolved in diluted acid solutions.
Wet digestion
for elemental analysis involve the chemical degradation of sample matrices in solution, usually with a combination of acids to increase solubility in either Open Vessels (OVD) or Closed Vessel (CVD).
▸Closed Vessel Digestion (CVD)
The most commonly used CVD (Chemical Vapor Deposition) technique is microwave-assisted acid digestion. This technique involves exposing a sample to a strong acid in a closed vessel and raising the pressure and temperature through microwave irradiation.
Limitation of Microwave Acid Digestion
- Microwave digestion requires the use of dedicated vessels specifically designed for this purpose, which can hold only a fixed amount of sample.
- There is a possibility of explosion and cracking of digestion tubes due to the simultaneous build-up of pressure and temperature.
- The sample digestion process cannot be observed during the procedure and can only be observed at the end of the process.
- Temperature control is possible only through the use of an inbuilt scanner.
- Sample vessels should be placed symmetrically, and the matrix should be highly homogeneous to ensure sufficient digestion.
▸Open Vessel Digestion (OVD)
Open Vessel Digestion (OVD), also known as Acid Digestion, involves the use of acids to break down samples in open containers or screw-top vials under low pressure, typically heated on devices like hot plates, hot blocks, or sand baths.
Among these, hot block digestion is widely applied in acid digestion, offering stable temperature control and high sample throughput, making it especially effective for processes requiring the simultaneous treatment of multiple samples. Hot blocks provide consistent, controlled heating, enhancing the reliability and reproducibility of digestion results. Additionally, graphite hot plates are frequently chosen for their superior heating uniformity, temperature precision, and expanded sample handling capabilities, making them ideal for a range of analytical procedures.
Graphite heating plate is a heating platform made of graphite that handles a large number of samples regardless the size, shape or material of vessels.
For test tubes, graphite hot blocks are also available as extensions to be added onto the plate and increase versatility.
Mars Graphite Hotplate – for Ultra Trace Metal Analysis
Mars Hotplate for metal digestion
Hot Block Digestion for Trace Metal Analysis
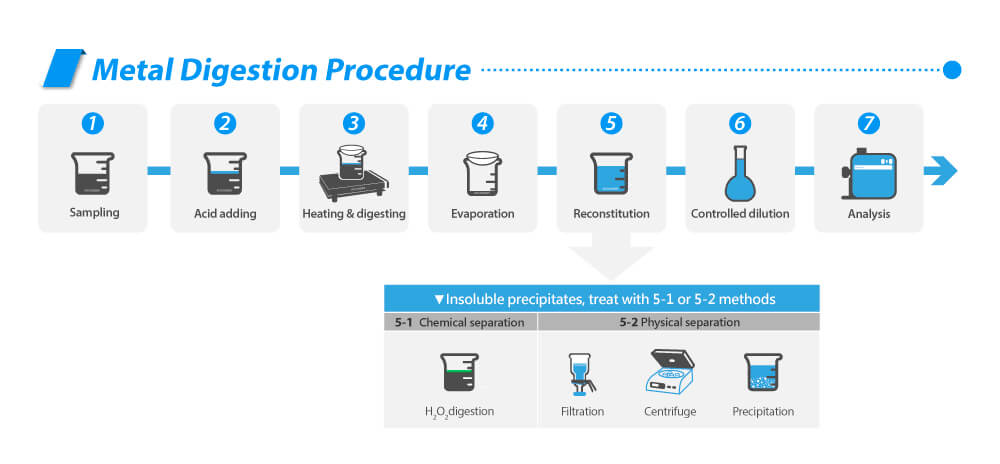
Hot Block Digestion Procedure▸
The process of acid digestion with a hot plate or hot block can be summarized as follows: Sampling, Acid Addition, Heating, Evaporation, Reconstitution, Controlled Dilution, and Analysis.
For insoluble precipitates, they can be treated with H2O2 digestion or removed through filtration, centrifugation, or precipitation.
For insoluble precipitates, treat with H2O2Digestion, or Filtration, centrifuge or precipitation to remove.
You may also be interested in
Vacuum Filtration
Chemker Chemical-resistant Vacuum Pump
Comparison of CVD & OVD
|
|
Closed Vessel Digestion |
Open Vessel Digestion |
|
Container |
Dedicated vessels for microwave (PFA、TFA) |
PP, borosilicate glass, PTFE vials or flasks |
|
Handling Capacity |
Less |
More |
|
Safety Risks |
Risk of explosion |
Overheat |
|
In-Process Visibility |
No |
Yes |
|
Limitation |
More |
Less |
|
Risk of Cross-contamination |
High |
Low |
|
Cost of consumables |
More |
Less |
Common acids used for metal digestion
Commonly used acids for metal digestion in trace metal analysis procedures include nitric acid (HNO3), hydrochloric acid (HCl), hydrofluoric acid (HF), hydrogen peroxide (H2O2), perchloric acid (HClO4), and sulfuric acid (H2SO4), among others.
The detailed characteristics of these acid varieties are listed below.
|
Variety |
Characteristics |
|
Nitric acid (HNO3) |
• Strong oxidizing properties |
|
Hydrochloric acid (HCl) |
• Strong oxidizing properties |
|
Hydrofluoric acid (HF) |
• Dissolution of some metals (eg: silicon, titanium, tungsten, platinum, etc.) |
|
Hydrogen peroxide (H2O2) |
• Can assist in the conversion of carbon to CO2 and increase the digestibility |
|
Perchloric acid (HClO4) |
•Rapid reaction with inorganic carbon |
|
Sulfuric acid (H2SO4) |
• Sample carbonization |
References:
1) Ashing – an overview | ScienceDirect Topics
2) SAMPLE DISSOLUTION FOR ELEMENTAL ANALYSIS | Wet Digestion – ScienceDirect
3) Benefits of Microwave Digestion over Open Acid Digestions (lab-training.com)
4) Sample Digestion Methods – ScienceDirect



